
His model suggested that most of the mass of the atom was contained in the small nucleus, and that the rest of the atom was mostly empty space. Following his famous gold leaf experiment Read More What was the name of Ernest Rutherford atomic theory? It is because of the outcome of the famous alpha particle scattering experiment done by some other scientists Read More Who is the scientist that a positive charge atom must be concentrated in a small region called the nucleus? Read More How did Rutherford know that there was something at the center of the atom? The element Rutherfordium (element 104) is named after Sir Ernest Rutherford who discovered the structure of the atom. Read More Who is a famous New Zealand person with a chemical named after them?
Martin Luther King Jr., Mother Trease, Ernest Rutherford, Nelson Mandela, Jimmy Carter, and many other famous people. Never heard of Rutherford famous gold foil experiment? Read More Who are the famous people who won nobel prize? Read More Would particles pass straight through gold foil?
#Rutherford experiment conclusion full
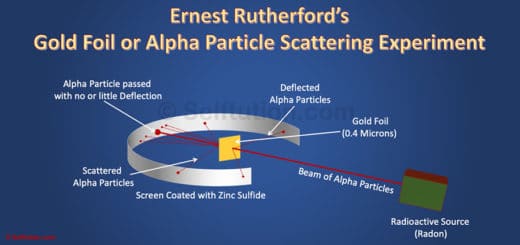
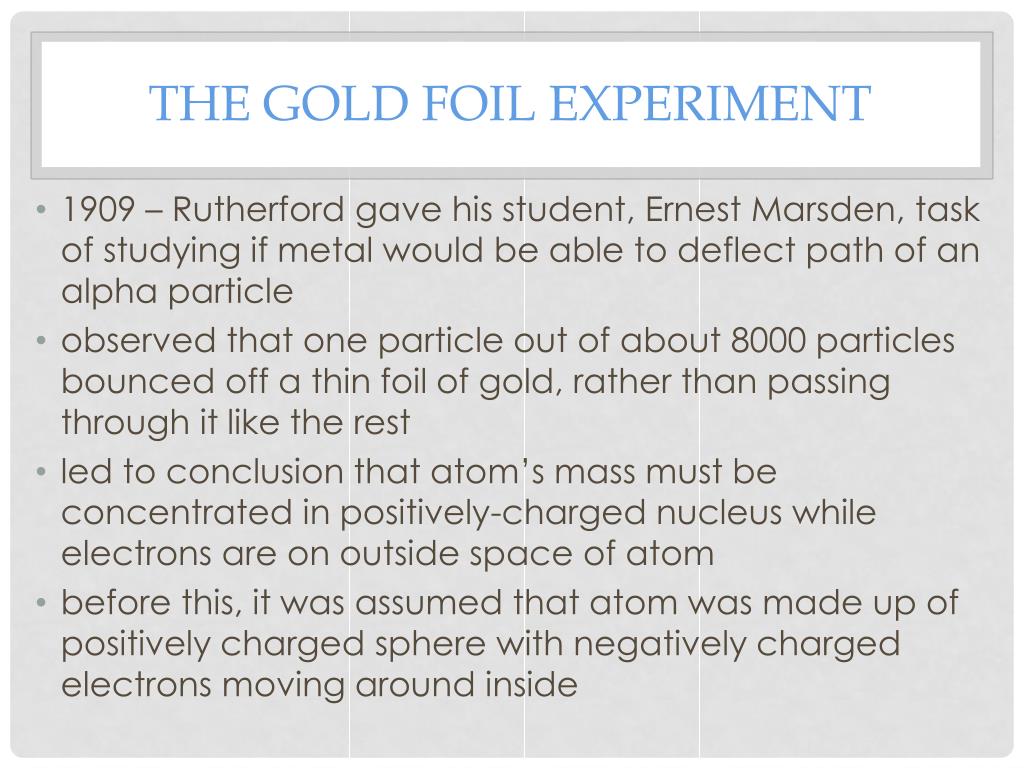
Read More What is Rutheford's full name?Īssuming you're talking about the famous chemist/physicist, his full name was Lord Ernest Rutherford (1871-1937). Read More Who discovered that there is a small dense positively charged nucleus in an atom?ĭmitri Ivanenko proposed the idea of a neutron and proton nucleus in 1932, the nucleus was itself discovered in 1911 by Ernest Rutherford while reviewing the famous Geiger-Marsden experiment of a few years previous. He was the first person to 'split the atom'. Read More Which New Zealander became famous for splitting the atom?Įrnest Rutherford was the man you are thinking of. Read More Why was Ernest Rutherford famous?īecause he proposed an atomic model for elements and he expressed that electrons turns around the proton in circuits. Read More How did Ernest Rutherford's gold foil experiment change the model of the atom?Īfter that famous experiment, it was realized that atoms were mostly empty space. In the early period of the 1900s, Ernest Rutherford became famous for his discovery of the nucleus. Sir edmund Hillary Kate Sheppard ernest rutherford Read More Who identified the nucleus? Read More Famous Settlers to New Zealand? Read More What did Thomas Rutherford discover about the atom?ĭo you mean Ernest Rutherford? Because he is famous for discovering the structure of an atom's nucleus without even looking at it. in 1909 Read More Who discover the nucleus using the alpha particles bombarded into the gold leaf?Įrnest Rutherford conducted this famous Gold-foil experiment - well he supervised Marsden and Geiger performing it which lead to the planetary model of the atom. If your thinking of the famous Gold Foil Experiment then he conducted it in 1911. Read More What year did Rutherford do his experiment? Rutherford supervised the experiment in his famous beta particle scatter experiment with gold foil, so he is given credit. Read More Who used polonium and gold foil in his famous experiment? Rutherford has demonstrated that the atomic nucleus is positive and separate from the electrons. his full name was lord ernest Rutherford Read More What did Rutherford expect to happen in his famous experiment? Rutherford was a famous Russian scientist who discovered nucleus in chemistry. Let m and v be the mass and velocity of the α-particle directed towards the centre of the nucleus.Scientist who is famous for his gold foil experiments? Who is rutheford? The distance of α-particle from the nucleus in this stage is called as the distance of closest approach and is represented by r 0. When whole of the kinetic energy is converted into electrostatic potential energy, the α-particle cannot further move towards the nucleus but returns back on its initial path i.e α-particle is scattered through an angle of 180°. Īs the α-particle approaches the nucleus, the electrostatic repulsive force due to the nucleus increase and kinetic energy of the alpha particle goes on converting into the electrostatic potential energy.

An α-particle which moves straight towards the nucleus in head on direction reaches the nucleus i.e., it moves close to a distance r 0.


 0 kommentar(er)
0 kommentar(er)
